During the Covid-19 pandemic, the need for medical devices is increasing. However, the production of medical devices in Indonesia is still imported as much as 55%. Not only that, sometimes Indonesian producers buy imported production materials, while the assembly is done in the country.
So to overcome this, the government is aggressively optimizing the Level of Domestic Capability (TKDN) to manufacturing production efforts funded by the state. This is to encourage productivity capabilities and competitiveness among national industries against world trade that tends to be closed.

The government finally issued regulations on TKDN regulated in The Minister of Industry Regulation No. 29 of 2018. The regulation contains the provisions and procedures for calculating the value of the domestic capability level (TKDN). TKDN regulation is prioritized for several sectors, one of which is the medical device industry which will later spur in terms of components and electromedical.
For the medical device industry, the existence of TKDN can encourage product marketing to foreign markets and increase local products. The medical device industry needs to have a TKDN certificate. This short article will be explained about TKDN. Here’s the full view.
Definition of Domestic Capability Level (TKDN)
Domestic Capability Level (TKDN) is the magnitude of the components of domestic production in goods, services, or combined between the two. The pupose of TKDN by the government is as follows:
- The use of domestic production is increasing.
- The level of effeciency of the national utilization industry is expected to be able to compete in world market.
- Opening jobs.
- The country’s deviation is becoming more frugal.
- Reduce dependence on external products.
In the medical device sector, the Ministry of Industry targets 43% in 2021 will rise to 50% in 2024. While for the value TKDN in the health sector is at least 40% which includes work tools, working capital, and labor.
TKDN Certificate Function

The TKDN certificate owned by the company providing goods/services serves as a plus to get a large enough credit/point in following the procurement of goods. In addition, TKDN certificates also provide waivers in fiscal facilities. This applies only to certain products. The issuance of TKDN certificates for goods/services providers means that the government gives import permits to support the production process.
TKDN Certificate Submission Flow

To obtain the TKDN certificate, the company providing goods/services can submit to an independent TKDN institution appointed by the ministry of industry, namely PT. Sucofindo and PT. Surveyors. Manufacturing companies that produce medical devices that want to apply for a TKDN certificate can follow the flow of submission as follows:
- The company providing goods/services conducts Self Assessment. The company providing goods/services must fill out the files and administrative requirements as submission of TKDN certificates sent to independent TKDN institutions.
- Independent verification agency TKDN accepts certification application from goods/services provider companies.
- The surveyor team from TKDN Institute conducted field surveys to companies and assessments based on Self Assessment data submitted by goods/services providers.
- The Independent Institute of TKDN makes the final report of the TKDN assessment. After the surveyor team conducts an audit of the company, then make the results of an assessment of the company.
- The Independent Independent Institute of TKDN makes a TKDN or BMP report and submits it in the draft TKDN online.
- The P3DN Centre received the report and held a meeting of the TKDN/BMP report discusussion panel.
- If the report is approved by the P3DN center.
- The certificate is submitted to the TKDN Independent Verification Agency.
- The certificate is received and then submitted to the company providing goods/services.
- Then the company providing goods/services has been.
The process of applying for TKDN certificates usually takes less than 3-4 months. This process also depends on the completeness of the company’s data. If the company data is complete, then the certification process will be processed immediately. While the announcement of TKDN certificate f can be seen through the official website of the Ministry of Industry.
Solo Abadi Produces Health Products With TKDN Certificate
Solo Abadi is a manufacturing company that produces medical devices, where production materials, means of production, and human resources come from within the country. So to support government programs in TKDN optimization, Solo Abadi has applied for a TKDN certificate. By obtaining a TKDN certificate, Solo Abadi can improve the optimization of natural resources and human resources in the country.
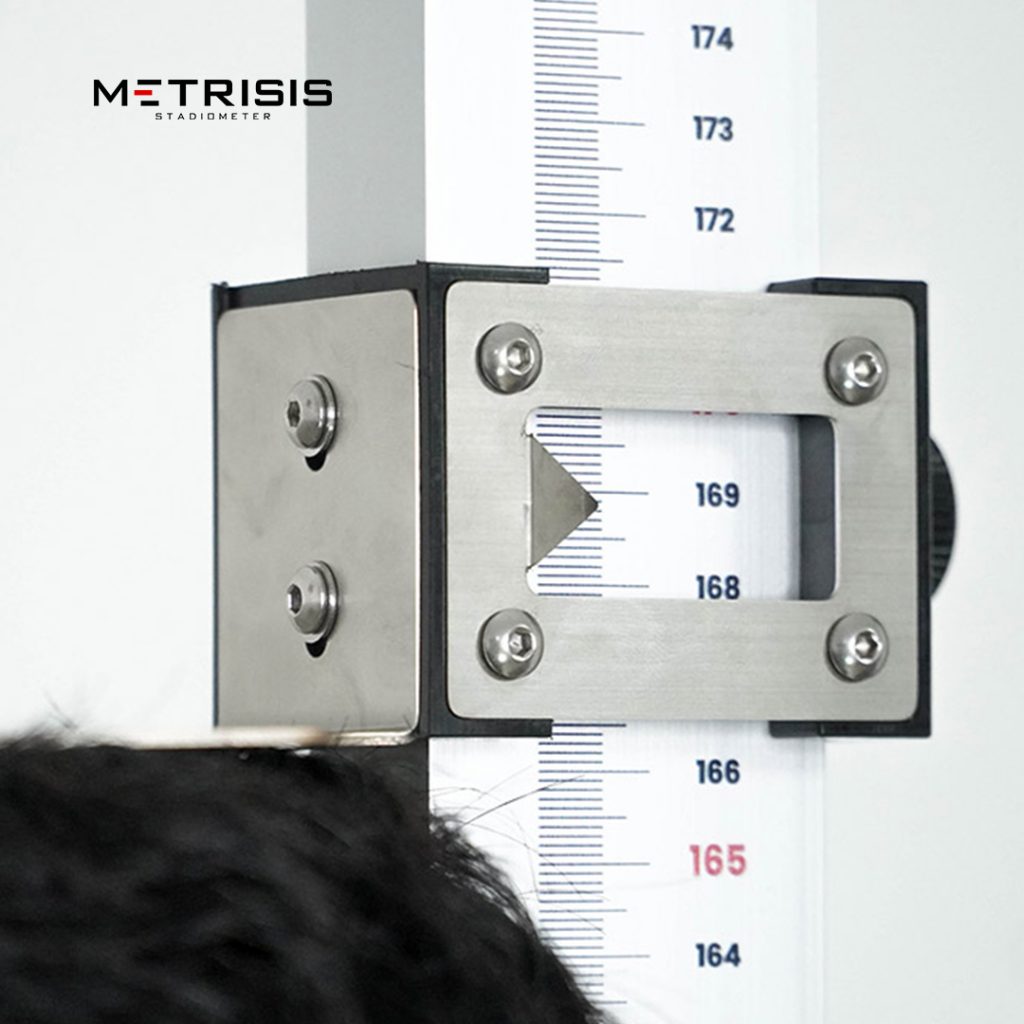
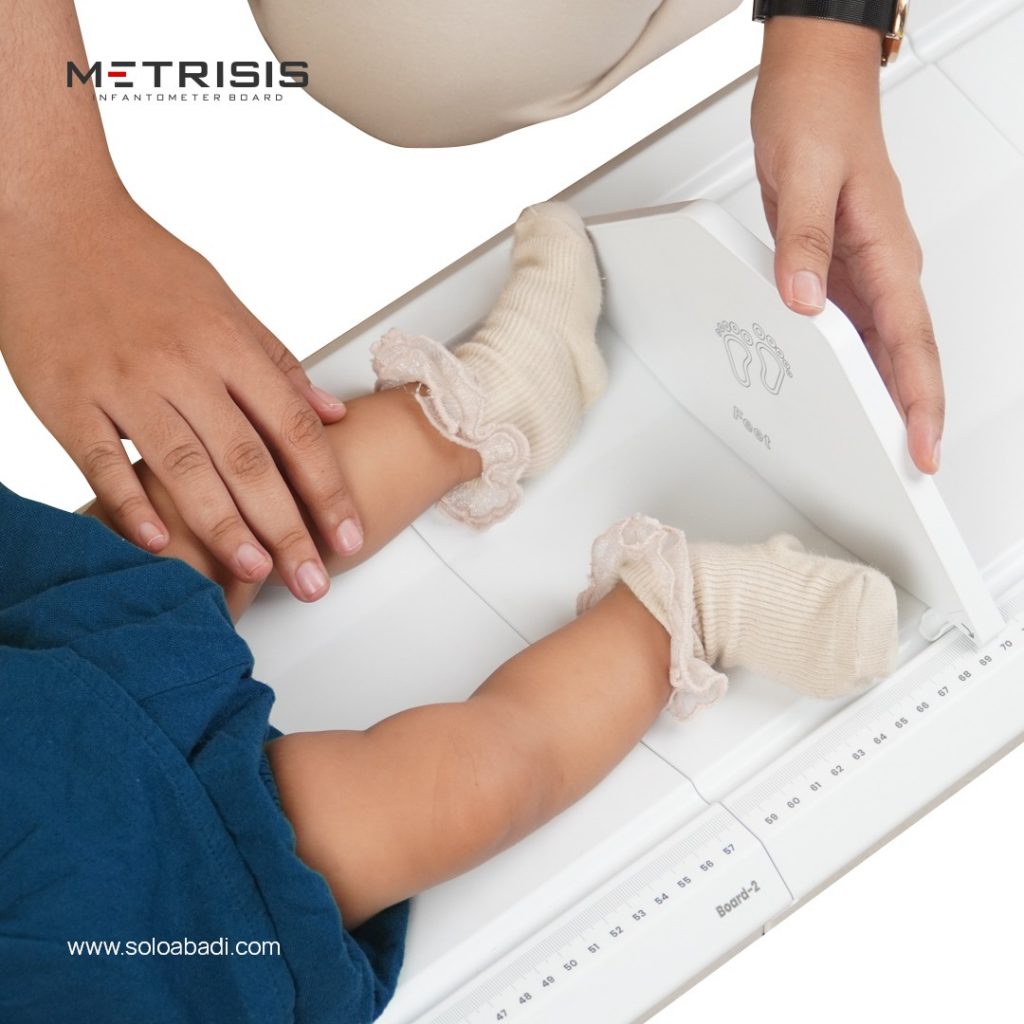
By bagging the TKDN certificate, Solo Abadi can import into the global market to market genuine health products made in Indonesia. Health products produced by Solo Abadi include anthropometric chairs, metritis, and stunting kits.
Read also articles about 5 Reasons Why You Should Buy Medical Devices From Domestic.