During the Covid-19 pandemic, the need for medical devices is increasing. This certainly affects import activity to neighboring countries and foreign countries to meet increasing health needs for the survival of many people. Whereas medical device manufacturers in Indonesia itself can develop their products without having to import from outside.
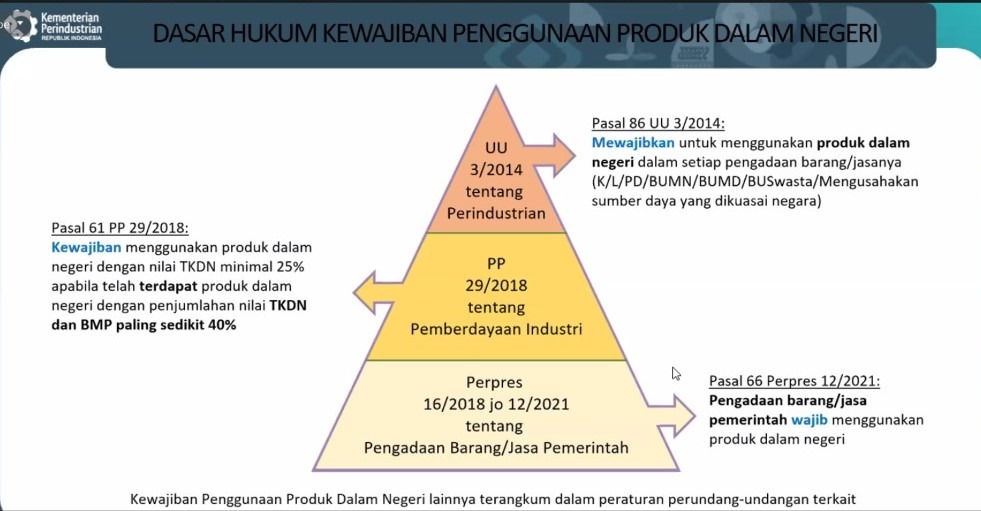
The government together with the Ministry of Industry issued regulations on the obligation to buy domestic products. This regulation stipulated in article 66 of Presidential Regulation 12/2021 on the procurement of that must use domestic products.
Domestic products are goods/services including the design and engineering produced or done by companies that invest or produce in Indonesia using all or part of the Indonesian citizen’s workforce, and the process uses Raw Materials or components that all or parts come from within the country.
In the use of domestic products, the government encourages medical device manufacturers in the TKDN program. TKDN is the amount of domestic content in goods, services, and a combination of goods and services.
This TKDN program has been contained in article 61 PP 29/2018 on the obligation to use domestic products with a TKDN value of at least 40%. The use of domestic products that already have TKDN of at least 40% means that it must be purchased and used in the procurement of goods/services in the government, so import activities are prohibited.
Domestic products that have reached TKDN must be certified as proof that the product is a local product. The TKDN certification was issued by the Ministry of Industry for local medical device manufacturers. By 2022 the government is targeting TKDN certification for health products to reach 9,000 products. This is because medical device products become a priority in the pandemic period.
Why TKDN certified?
The Ministry of Industry issued regulations on TKDN certification in domestic products aimed at encouraging all products produced and produced domestically to include procurement projects of goods/services. More detailed TKDN certification has other purposes including:

- Increase the use of domestic production
- Increase employment opportunities
- Increase national utilization and improve industrial efficiency so that it can compete in the world market
- Saving the country’s foreign exchange
- Reduce reliance on overseas products through optimization of government spending
Therefore, domestic medical device products that already have TKDN certification must be purchased and used in the procurement of government goods/services. That way they increase in medical device manufacturers in Indonesia can be independent without having to import. Medical device manufacturers in Indonesia are also allowed to carry out export activities to increase the country’s foreign exchange.
TKDN Certified Alkes Products from Solo Abadi
PT Solo Abadi Indonesia is one of the medical device manufacturers in Indonesia that moves to produce measuring devices such as portable stadiometers, infantometer boards, and portable infantometers. These products currently have TKDN certification and pass calibration tests. Portable stadiometer from Solo Abadi itself has the highest TKDN score of 69.27%. Thus, products from Solo Abadi have met the standards and criteria for use in health facilities, both in health centers and procurement of goods in government.

Portable Stadiometers 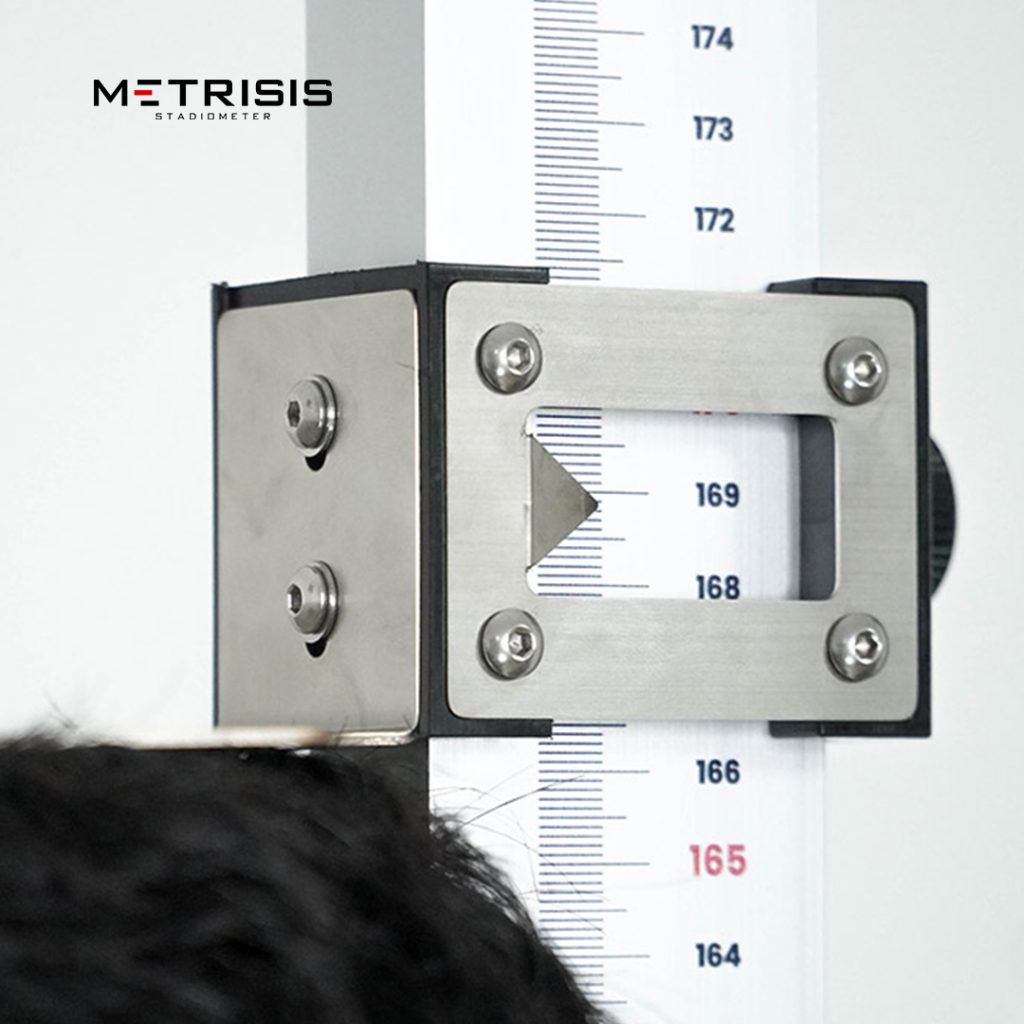
Portable Stadiometers 
Portable Stadiometers 
Infantometer Boards 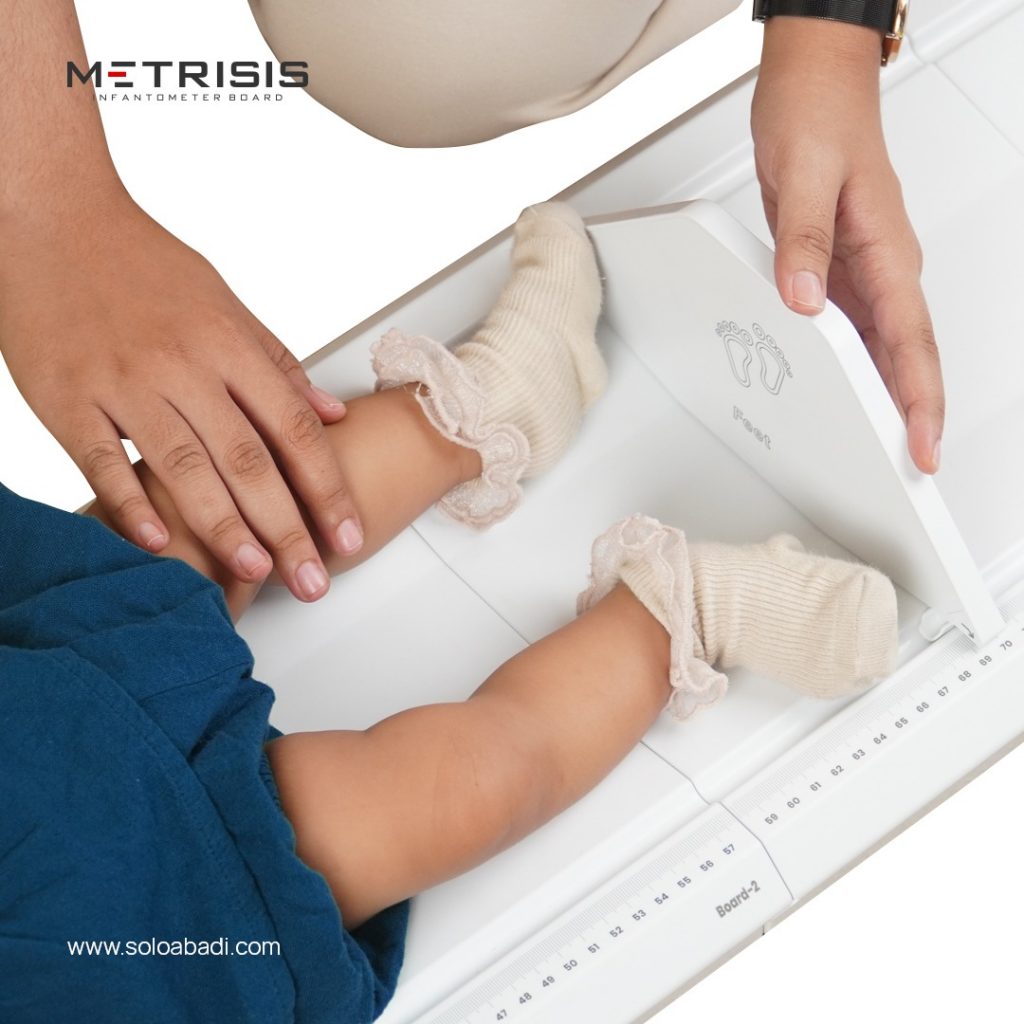
Infantometer Boards 
Infantometer Boards 
Portable Infantometers 
Portable Infantometers 
Portable Infantometers
For those of you who are interested in seeing more about products from Solo Abadi, just contact us via WhatsApp. If you are interested in buying directly fill out ASK FOR PRICE through the available link. Follow us on Instagram, Facebook, and YouTube Solo Abadi to get the latest information from us.
Read the next article on the TKDN Encourages The Production of Medical Devices