Surakarta, PT Solo Abadi Indonesia — Wednesday (9/2) PT Solo Abadi Indonesia participated in the socialization of the Registration of Medical Device And PKRT Licenses organized by the Directorate of Assessment of Medical Devices and PKRT, and the Ministry of Health of the Republic of Indonesia. This socialization is done through a zoom video conference meeting from 09.00-12.00. This socialization was attended by approximately 1000 participants from medical device manufacturers in Indonesia.
The purpose of this socialization is to accelerate the Implementation of Risk-Based Licensing Regulation Evaluation through trials of the integration of the medical device licensing system and PKRT with the OSS RBA method. OSS RBA is an Online Single Submission Risk-Based Approach used in business licensing given to business actors to start and carry out their business activities to be assessed based on the level of risk of electronically integrated business activities. The results of the OSS RBA trial are used in permits for new applications for medical device businesses and imported PKRT and the creation of new applications for medical device permits and domestic PKRT.
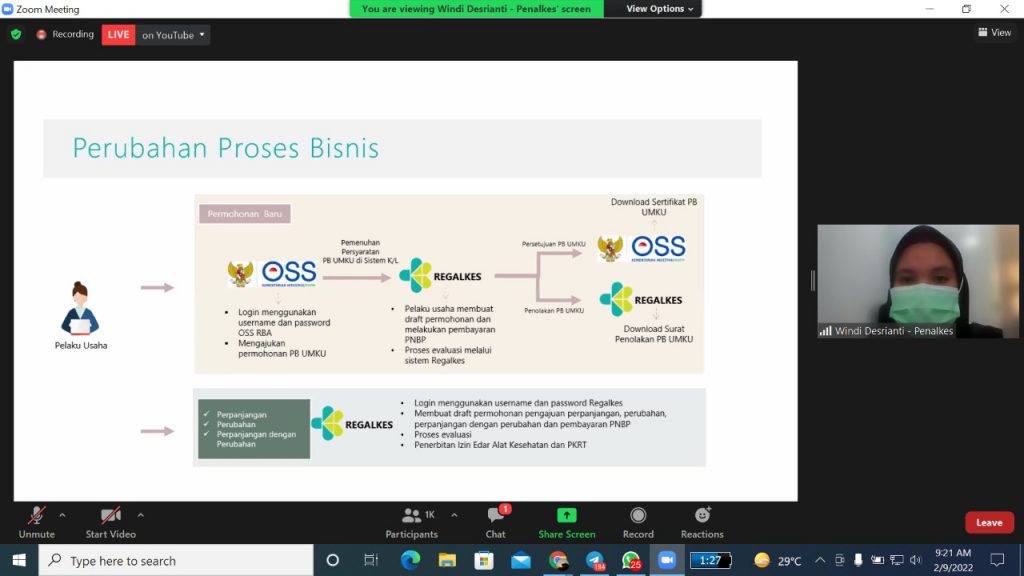
This socialization is packed with discussion sessions, so many participants ask technically related questions from the use of the RBA SSO. Before the launch of this RBA SSO, medical device distribution permits were carried out in the registration of medical devices (regalkes) to obtain a certificate of distribution of medical devices (SDAK). The RBA SSO system is used for the submission of new business actors registering medical device distribution permits. Meanwhile, manufacturers who have registered distribution permits through regalkes do not need to make new applications again but can wait for the latest system to extend or change the distribution permit of medical devices.
The Directorate of Medical Device Assessment and PKRT also explained about the flow of licensing applications seeking the distribution of medical devices through the RBA SSO is as follows:
- Business actors are registered in OSS with the appropriate KBLI
- Business actors apply through oss.go.id and complete all requirements.
- Furthermore, the Ministry of Health will verify applications that are integrated with the OSS RBA system with the SSO mechanism.
- After verification is approved by the Ministry of Health, it will be issued a certificate of medical device distribution permit to business actors that can be downloaded through OSS and email the permit to the certification of wasales@gmail.com.
This OSS RBO system has been in effect since February 7, 2022. So for medical device manufacturers who have not registered a distribution permit to immediately register medical devices. If there are obstacles in registering or applying for a distribution permit can contact the Directorate of Assessment of Medical Devices and PKRT. The medical device distribution license certificate is valid for 5 years from the time the certificate is issued. After the permit period is up, businesses can apply for an extension.
PT Solo Abadi Indonesia as a manufacturer of anthropometric medical devices has had a medical device distribution permit (IPAK) since 2021. Registration of this circular permit is done through the regalkes website and has obtained a circular permit certificate by the directions of the Directorate of Medical Device Assessment and PKRT. Thus all medical device products from PT Solo Abadi Indonesia have pocketed medical device distribution licenses (IDAK) valid from 2021 to 2026.
participants in socialization of medical device distribution permits 
opening of socialization of medical device distribution permits from the Directorate of Medical Device Assessment and PKRT 
Welcome by Sadikin Sadeq of Farmalkes
About PT Solo Abadi Indonesia
PT Solo Abadi Indonesia is a company that located in Surakarta, Central Java. Focused in manufacturing field and is the biggest measuring tool producer in Indonesia established since 2005. Products by Solo Abadi such as anthropomethric chair and portable anthropomethry is applicable in several studies, such as health, anthropology, sports, forensic, industrial engineering. Solo Abadi also supports stunting prevention from an early age by providing stunting kit products to monitor baby growth and development, one of the products that is a part of stunting prevention efforts is an infantometer board to measure infants aged from 0 to 2 years.
For further information you could contact our email admin@soloabadi.com or through our WhatsApp 08510888111