Surakarta, PT Solo Abadi Indonesia — As a domestic medical device manufacturer, of course, PT Solo Abadi Indonesia always prioritizes quality and quality in every product. PT Solo Abadi Indonesia is a member of the Indonesian Medical Device Manufacturers Association (ASPAKI) to obtain various information and activities about medical device manufacturers throughout Indonesia.
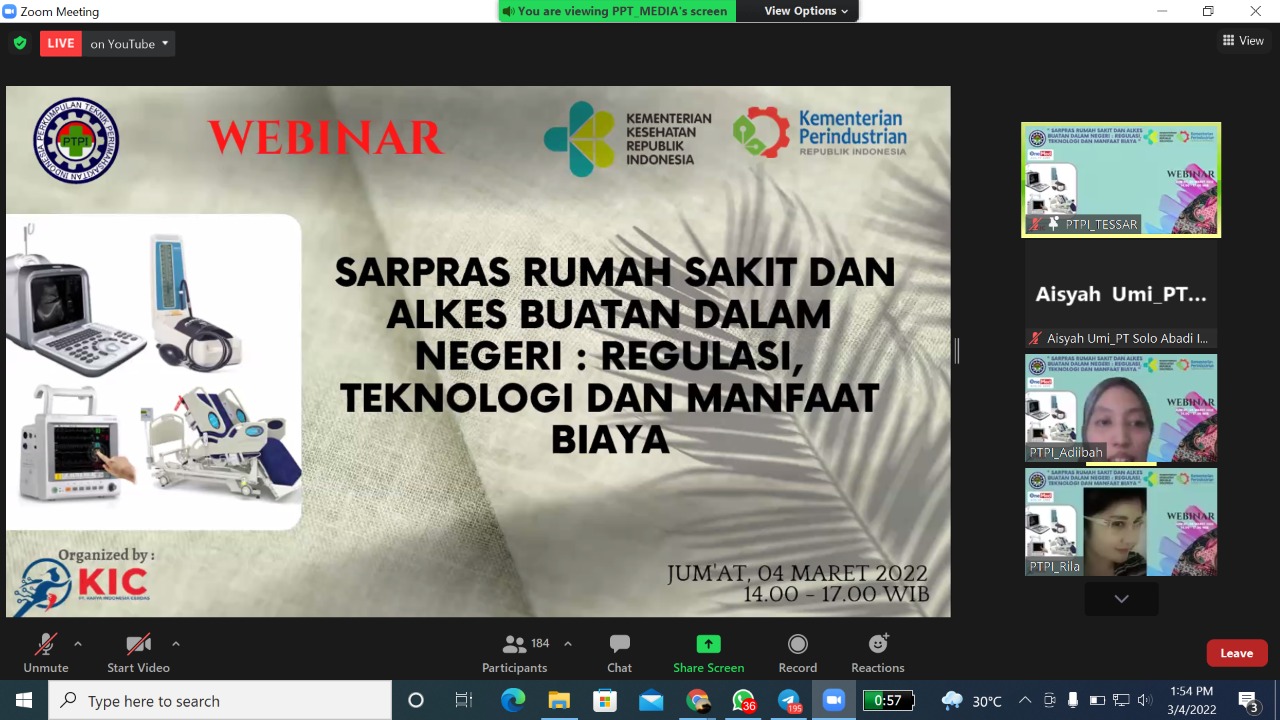
On Friday (4/3/2022), PT Solo Abadi Indonesia again participated in the webinar Sarpras Hospital and Domestic Medical Devices: Technology Regulation and Cost Benefits organized by the Indonesian Hospital Engineering Association (PTPI). Webinar held through video conference zoom meeting was attended by approximately 425 participants. Attended by actors and manufacturers of the medical device industry, hospitals and health centers, as well as researchers, lecturers, and educational institutions.
This webinar lasts for 3 hours starting at 14.00 WIB and ending at 17.00 WIB. In this webinar presented five speakers namely Nila Kumalasari, ST, MT, Ir. Sodikin Sadek, M.Kes, drg. Iing Ichsan Hanafi., MARS, Ade Tarya Hidayat, and dr. Jemmy. The webinar began with the opening by PTPI president Prof. Dr. Ing Eko Supriyanto. Each source presents material about the improvement, quality, and use of domestic medical devices.
Head of the Ministry of Industry’s Domestic Product Improvement Center, Nila Kumalasari, ST, MT revealed that the government targets domestic product and MSME spending in 2022 of Rp 400 trillion. This is by the President’s directive regarding domestic products that have been regulated in government regulation No. 29 of 2018 on industrial empowerment.
“Every domestic product, both in the form of goods and services must have TKDN (Domestic Component Level) which is the amount of domestic content, and BMP (Weight of Corporate Benefits) which is the value of awards given to industrial companies that invest and produce in Indonesia,” said Nila Kumalasari when presenting webinar material.
He also revealed that domestic products must be purchased if they already have TKDN and BMP of 40%. “Domestic products must be purchased if they already have TKDN of 25% and AMP weight of 15% or all of them by 40%, so imported products are prohibited,” he added.

During the question and answer session, a representative from PT Solo Abadi Indonesia, Hanif Bramasta Ibrahim asked Nila Kumalasari a question.
“What are the further actions regarding the government that has uploaded KAK/procurement of goods on the SIRUP website with foreign products? Whereas for the manufacture of these goods there are already products with TKDN above 40%,” he asked.
Then, Nila Kumasari replied that every KLDP, BUMN, and BUMD for procurement of goods/services has been directed to enter or input in the mandatory category of domestic products. Regarding SYIUP, it is currently not fully implemented. For this reason, Nila Kumalasari also wants input and advice from various industry sectors in the improvement of the SIRUP website so that it can be used this year.
PT Solo Abadi Indonesia is one of the domestic manufacturers that produce medical devices in the form of stunting and anthropometric products that already have TKDN certification above 40%. For portable stadiometer products have the highest TKDN certification of 69.27%. This has been above the achievement of TKDN targets by the direction of the government.
With full firmness, PT Solo Abadi Indonesia fully supports government policies and programs in improving domestic products through this TKDN certification. PT Solo Abadi Indonesia also expects support and invites the government and also the people of Indonesia to buy domestic products, especially medical devices.

About PT. Solo Abadi Indonesia
PT. Solo Abadi Indonesia is a company located in Surakarta, Central Java. Engaged in manufacturing and is the largest producer of Portable Stadiometer, Infantometer Board, Anthropometry Chair and Stunting Kit in Indonesia, which was established in 2005. Solo Abadi products can be applied in various fields of science such as health, anthropology, forensics, industrial engineering, product design, academia, to the military. We continue to advancing the domestic industry by delievered certified and licensed products.
For further information you could contact our email admin@soloabadi.com or through our WhatsApp 08510888111